The Calphad module is a thermodynamic calculation module based on Open Calphad developed by Professor Bo Sundman.
Using this module, you can calculate the kind of the phase in a microstructure of alloys due to the heat-treatment temperature, the amount of the phase, the component in the phase, heat-treatment information for the microstructure, etc.
The Calphad module gives nine services.
- You can calculate the information of each phase according to the temperature condition.
- You can calculate the phase diagram between two elements.
- You can calculate the phase diagram between three elements.
- You can calculate each phase information of the microstructure of alloy with various elements added according to the temperature.
- You can calculate energy function (G, H, S, activity, etc) according to the temperature or composition. If you want to know the types of function information you can get, please refer to the Calphad Function Diagram section.
- You can calculate the phase information of alloy according to the custom settings.
- From the matrix phase, you can calculate driving force that represents the degree of appearance of other phases.
- You can stacking fault energy for austenite steels and Ni-base alloys.
- You can antiphase boundary energy for Ni-base alloys involving Ni3Al precipitations.
To perform better thermodynamic calculation, the most importance is the thermodynamic database.
It is important how well the multi-component database describes binary and ternary information. Generally, if the database describe well the binary and ternary information, can expand to multi-component. Therefore, user must check the binary and ternary information of the database, and use the database suitably.
We trying to offer a better database. Please keep in the mind differing the calculation cost to obtain the phase diagram result according to the quality of the database.
- MatSQ Ni 1.0 ($ 2.00 / Run)
- MatSQ AL 1.0 ($ 1.00 / Run)
- MatSQ FE 2.0 ($ 2.00 / Run)
- MatSQ HEA 2.1 ($ 2.00 / Run)
- Binary system (Academic) ($ 0.01 / Run)
- Fe-Cr-Mo-Si-V-C system (Academic) ($ 0.01 / Run)
▲
This database is a commercial database for Ni-base systems.
Al < 10 | Co < 25 | Cr < 20 | Fe < 20 | Mo < 7 | Nb < 5 |
Ta < 7 | Ti < 5 | V < 2 | W < 8 | C < 0.1 | N < 0.1
This database is a commercial database for Ni-base systems.
Al < 10 | Co < 25 | Cr < 20 | Fe < 20 | Mo < 7 | Nb < 5 |
Ta < 7 | Ti < 5 | V < 2 | W < 8 | C < 0.1 | N < 0.1
LIQUID, FCC_A1, BCC_A2, HCP_A3, MX_FCC, DELTA_NI3NB, GAMMA_DP, GAMMA_PRIME, ETA, LAVES, MU_PHASE, SIGMA, DELTA_NIMO, NIAL, P_PHASE, R_PHASE, M23C6, M3C2, M5C2, V3C2, AL4C3, KAPPA, KSI_CALRBIDE, CEMENTITE, CR2VC2, M6C, M12C, M7C3, MC_ETA, MC_SHP, CO3V, COV3, CUB_A15, GRAPHITE, ALN, CRNBN, PI, TI2N, FE4N, Z_PHASE, NI2CR, GAS
▲
This database is a commercial database for Al-base systems.
Range of alloying elements (mass percent)
Cr < 5 | Cu < 5 | Fe < 5 | Li < 10 | Mg < 5 | Mn < 5 |
Ni < 1 | Sc < 5 | Si < 10 | Ti < 1 | Zn < 5 | Zr < 1 |
This database is a commercial database for Al-base systems.
Range of alloying elements (mass percent)
Cr < 5 | Cu < 5 | Fe < 5 | Li < 10 | Mg < 5 | Mn < 5 |
Ni < 1 | Sc < 5 | Si < 10 | Ti < 1 | Zn < 5 | Zr < 1 |
LIQUID, FCC_A1, BCC_A2, HCP_A3, HCP_ZN, CBCC_A12, DIA_A4, AL11CR2, AL13CR2, ALCRCU_TAU, TAU_ALCUZN, AL18CR2MG3, AL9CR3SI, ALCRSI_T3, AL13CR4SI4, AL58CR31SI11, OMEGA, AL23CUFE4, ALCUMN_T1, ALLI, ALLIMG_TAU, ALMG_BETA, AL13FE4, ALCRFEMNSI_A, ALFEMNSI_B, ALFENI_T, ALCUSC_TAU, ALFESI_T6, AL12MN, AL3NI, L12_AL3X, AL3TI_H, AL3TI_L, AL3ZR_TETR, LAVES_C14, T_MGALCUZN, PI_PHASE, Q_PHASE_L, Q_PHASE_H, S_PHASE, AL2CU_THETA, AL2MG5SI4_BP, ALCRSI, ALMGZN_GP, ALMG4ZN11, AL6MN, BETA_DP, B_PRIME_L, B_PRIME_H, ALMGZN_ETA_P, PRE_B_DP, AL6CU2_TH_DP, THETA_PRIME, U1_PHASE, ALSC2SI2, U2_PHASE, AL2CU3_D, ALCU_E, ALCU_ETA, ALCU_G_D83, AL9CU11_Z, ALCULI_T1, ALCULI_T2, ALCULI_TB, ALCUMG_V, ALCUZN_1, AL2F2, AL5FE2, AL5FE4, AL8FENBSI2, ALFENI_T2, ALFENI_T3, ALFESI_T1, ALFESI_T2, ALFESI_T3, ALFESI_T4, ALFESI_T5, ALFESI_T7, ALFESI_T8, ALFESI_T10, ALFESI_T11, AL12MG2CR, ALMGMN_T, AL4MN, AL8MN5, AL11MN4, ALMGSI_B, ALMNSI_D, AL3NI2, ALM_D019, AL3M_D022, ALSIZR_T1, ALSIZR_T2, AL4SI5ZR3, ALTI, AL2TI, AL11TI5, ALZNCU_G_H, AL3MG2_B, AL30MG23_E, AL12MG17_GAMMA, ALCUMG_Q, ALLISI, SIGMA, H_SIGMA, LAVES_C15, LAVES_C36, MGSI_GP, MG2SI_B, MGSI_B_P, MG5SI6_B_DP, FESI, FESI2_H, FESI2_L, CU2MG_LAVES, CUMG2_LAVES, NIMG2_LAVES, NI2MG_LAVES, MGALZN_PHI, MGZN, MG2ZN3, MG2ZN11, SIZR_A, SIZR2, SI2ZR, SI3ZR5, SI4ZR5_A
▲
This database is a commercial database for Fe-base systems. In the present database, Co, Cu, and W have been added.
Range of alloying elements (mass percent)
Al < 5 | Co < 10 | Cr < 25 | Cu < 1 | Mn < 25 | Mo < 5 | Nb < 1
Ni < 20 | Si < 5 | Ti < 1 | V < 5 | W < 10 | C < 2 | N < 1 | S = trace
This database is a commercial database for Fe-base systems. In the present database, Co, Cu, and W have been added.
Range of alloying elements (mass percent)
Al < 5 | Co < 10 | Cr < 25 | Cu < 1 | Mn < 25 | Mo < 5 | Nb < 1
Ni < 20 | Si < 5 | Ti < 1 | V < 5 | W < 10 | C < 2 | N < 1 | S = trace
LIQUID, AL4C3, ALN, BCC_A2, CBCC_A12, CEMENTITE, CHI_A12, CRNBN, CUB_A13, DIAMOND_FCC_A4, FCC_A1, FE4N, FECN_CHI, GRAPHITE, HCP_A3, CO3MO, CO3V, CR3MN5, G_PHASE, KAPPA, KSI_CARBIDE, LAV_C15, LAV_C36, LAVES_PHASE, M23C6, M3C2, M3SI, M3V, M5C2, M5SI3, M6C, M7C3, M12C, WC, MC_ETA, MC_SHP, MSI, MU_PHASE, PI, P_PHASE, R_PHASE, SIC, SIGMA, TI2N, TI3AL, TI3N2, TI4N3, TIAL, V3C2, MNNI, MNNI2, CR2VC2, MN6N4, MN6N5, SI3N4, Z_PHASE, A_CHALC, ANILITE, B_CHALC, COVELLITE, CU2S, DISULF, DJURLEITE, FC_MONO, FC_ORTHO, FES_P, MNS_Q, PYRR
- Lee, Seok Gyu et al. 2020, “Effects of Cr addition on Charpy impact energy in austenitic 0.45C-24Mn-(0,3,6)Cr steels”, Materials Science and Technology, in press.
- Jafari, Majid et al. 2020, “Evolution of microstructure and tensile properties of cold-drawn hyper-eutectoid steel wires during post-deformation annealing”, Materials Science and Technology 41: 1-11
- Choi, Yong Hoon et al. 2019. “Influence of Initial Microstructures on Intercritical Annealing Behaviour in a Medium Mn Steel.” Materials Science and Technology 35(17): 2092–2100.
- Song, Hyejin et al. 2018. “Improvement of Tensile Properties in (Austenite+ferrite+κ-Carbide) Triplex Hot-Rolled Lightweight Steels.” Materials Science and Engineering: A 730: 177–86.
- Jung, Seungmun et al. 2017. “Effects of Mn and Mo Addition on High-Temperature Tensile Properties in High-Ni-Containing Austenitic Cast Steels Used for Turbo-Charger Application.” Materials Science and Engineering: A 682: 147–55.
- Kim, Hyunmin et al. 2015. “Dynamic Tension–Compression Asymmetry of Martensitic Transformation in Austenitic Fe–(0.4,1.0)C–18Mn Steels for Cryogenic Applications.” Acta Materialia 96: 37–46.
- Sohn, Seok Su, Byeong-Joo Lee, Sunghak Lee, and Jai-Hyun Kwak. 2014. “Effect of Mn Addition on Microstructural Modification and Cracking Behavior of Ferritic Light-Weight Steels.” Metallurgical and Materials Transactions A 45(12): 5469–85.
- Sohn, S S, B.-J. Lee, S Lee, and J.-H. Kwak. 2013. “Effects of Aluminum Content on Cracking Phenomenon Occurring during Cold Rolling of Three Ferrite-Based Lightweight Steel.” Acta Materialia 61(15): 5626–35.
▲
This database is a commercial HEA database for the Co-Cr-Fe-Mn-Ni-V-Cu-Mo-Al-Si-Ti-C-N system.
This database is recommended for use above 973K (700℃)
Co, Cr, Fe, Mn, Ni, V, Cu, Mo, Al, Si, Ti-C-N
Some examples of what data can obtain by Calphad User-defined menu.
This example is useful when you want to know the state of each phase by temperature according to the amount of one element in a situation where different amounts of alloying elements are determined.
We will obtain the phase information by temperature according to the amount of Cr when 8wt.% Ni.
The result is:
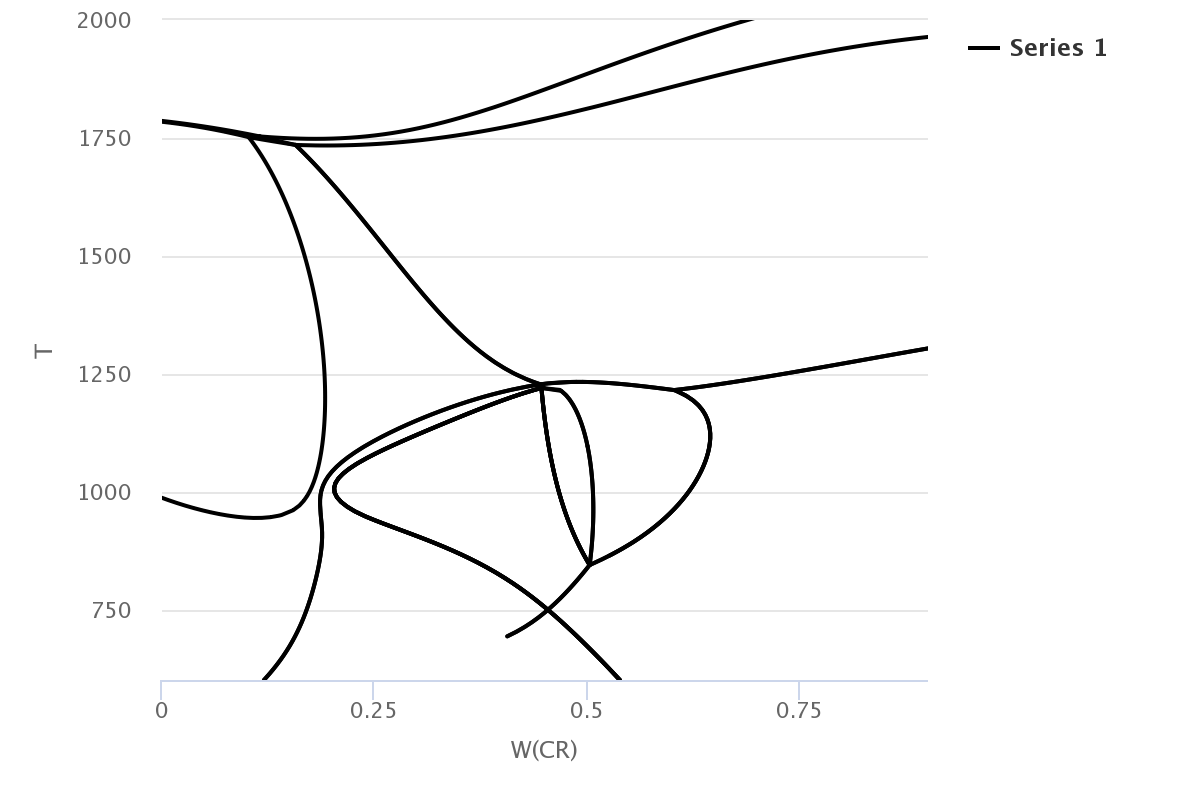 If you want to know the phase at 25 wt.%, 750 K, write that conditions as an initial parameter at the [List-equilibrium] tab. Getting the phase information of that condition by real list-equilibrium graph, we can find out the Fe-rich bcc phase exists 75.6%, Cr-rich bcc phase exists 11.8%, Fcc phase 10.9% exists.
If you want to know the phase at 25 wt.%, 750 K, write that conditions as an initial parameter at the [List-equilibrium] tab. Getting the phase information of that condition by real list-equilibrium graph, we can find out the Fe-rich bcc phase exists 75.6%, Cr-rich bcc phase exists 11.8%, Fcc phase 10.9% exists.
This example is want to find out the solubility line of VC carbide in austenite at the 10Mo-4.5Cr-0.1Si HS steel when the temperature is 1300 K.
The result is:
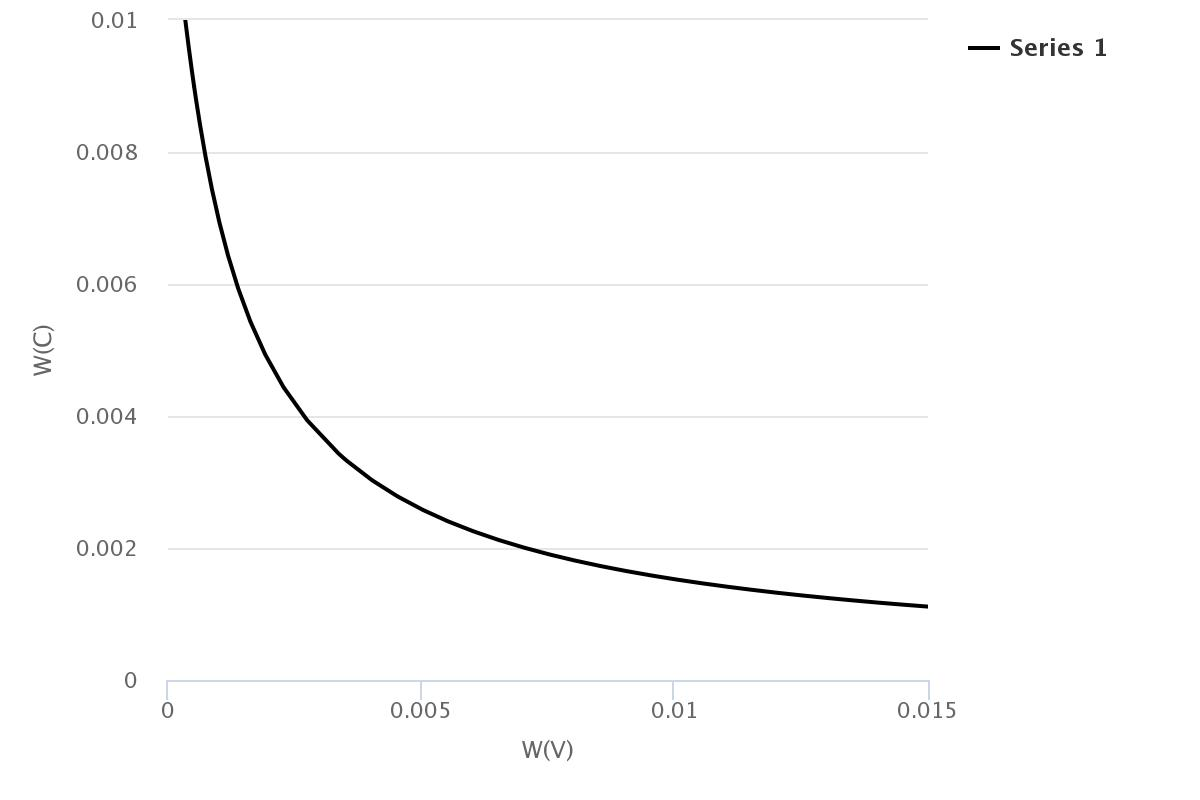
This result can analyze like the following figure by [List-equilibrium] tab.
We can check the crystallization amout of VC carbide exists in the Austenite according to how much the amount of V and C at the final.
This mode is used when you want to know the change of energy as the function of a specific phase or element.
In this way, functions can also be computed each other.
This database is a commercial HEA database for the Co-Cr-Fe-Mn-Ni-V-Cu-Mo-Al-Si-Ti-C-N system.
This database is recommended for use above 973K (700℃)
Co, Cr, Fe, Mn, Ni, V, Cu, Mo, Al, Si, Ti-C-N
1. Co-Cr-Fe-Mn-Ni + V system
2. Co-Cr-Fe-Mn-Ni + Cu system
3. Co-Cr-Fe-Ni + Mo system (with Co-Mo-Ni-V system)
4. Co-Cr-Fe-Mn-Ni + Al system
5. Co-Cr-Fe-Mn-Ni + Si system
6. Co-Cr-Fe-Mn-Ni + Ti system
7. Co-Cr-Fe-Mn-Ni + V + C system
8. Other systems (carbide, nitride systems, etc.)
2. Co-Cr-Fe-Mn-Ni + Cu system
3. Co-Cr-Fe-Ni + Mo system (with Co-Mo-Ni-V system)
4. Co-Cr-Fe-Mn-Ni + Al system
5. Co-Cr-Fe-Mn-Ni + Si system
6. Co-Cr-Fe-Mn-Ni + Ti system
7. Co-Cr-Fe-Mn-Ni + V + C system
8. Other systems (carbide, nitride systems, etc.)
- H. Kwon et al., High-density nanoprecipitates and phase reversion via maraging enable ultrastrong yet strain-hardenable medium-entropy alloy, Acta Mater. 248, 118810 (2023.04).
- D.G. Kim et al., Effects of deformation-induced martensitic transformation on quasi-static and dynamic compressive properties of metastable SiVCrMnFeCo high-entropy alloys, J. Alloys and Compds. 931, 167543 (2023.01).
- H. Chung et al., Doubled strength and ductility via maraging effect and dynamic precipitate transformation in ultrastrong medium-entropy alloy, Nature Communications 14, 145 (2023.01).
- H.-S. Do et al., A Novel High-entropy Alloy with Multi-strengthening Mechanisms: Activation of TRIP effect in C-doped High-entropy Alloy,Mater. Sci. Eng. A 859, 144220 (2022.11).
- H. Nam et al., Enhancement of tensile properties of gas tungsten arcwelds using Cu-coated CoCrFeMnNi filler and posteweld heat treatment, J. Mater. Res. & Techn. 19, 4857-66 (2022.07).
- H.-S. Do et al., A thermodynamic description for the Co-Cr-Fe-Mn-Ni system, J. Mater. Sci. 57, 1373-1389 (2022.01).
- Yang, Junha et al, “Effects of transformation-induced plasticity (TRIP)on tensile property improvement of Fe45Co30Cr10V10Ni5-xMnx high-entropy alloys”, Mater. Sci. Eng. A 772,138809 (2020.01).
- Wei, Daixiu et al. “Novel Co-rich high performance twinning-induced plasticity (TWIP) and transformation-induced plasticity (TRIP) high-entropy alloys”, Scripta Mater. 165, 39-43 (2019.05).
- Ondichoe, Ibrahim et al. “Experimental investigation and phase diagram of CoCrMnNi-Fe system bridging high-entropy alloys and high-alloyed steels” J. Alloys and Compds. 785, 320-327 (2019.05).
- Jo, Yong Hee et al. “FCC to BCC transformation-induced plasticity based on thermodynamic phase stability in novel V10Cr10Fe45CoxNi35-x medium-entropy alloys” Sci. Rep. 9, 2948 (2019.02).
▲
Thermodynamic information for several binary systems are provided as a free of charge to be used for educational purposes such as universities, institutions, and companies.
Al, B, C, Ce, Cr, Cu, Fe, Li, Mg, Mn, N, Nb, Nd, Ni, Si, Sn, Ti, V, Y, Zn, Zr
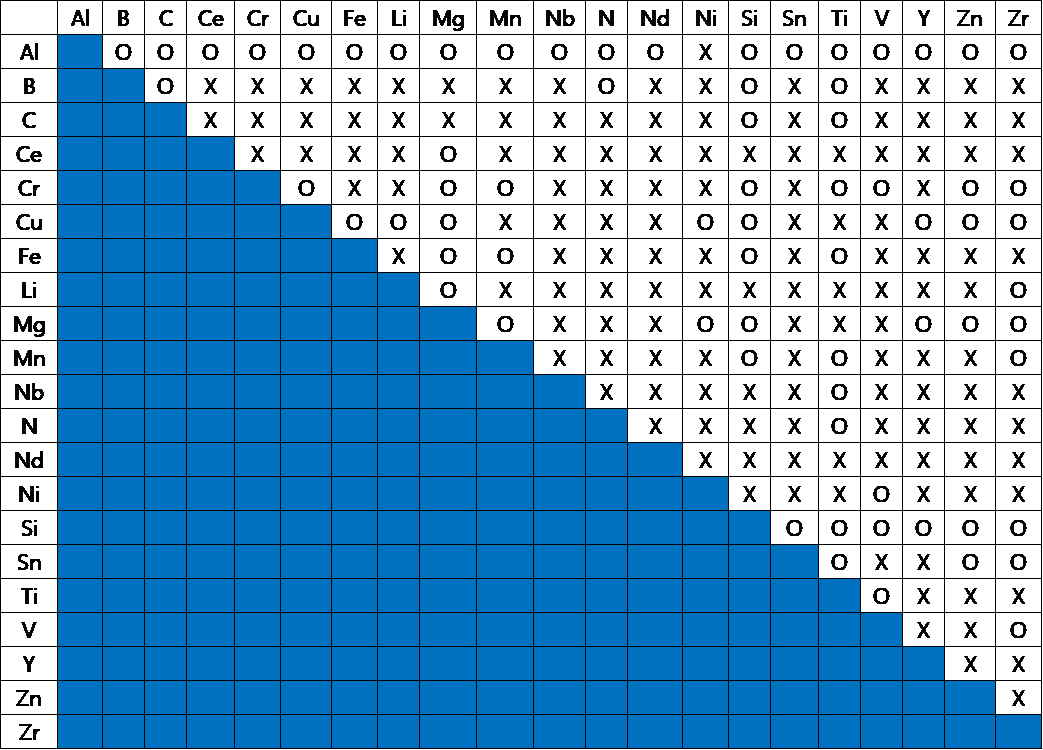
Thermodynamic information for several binary systems are provided as a free of charge to be used for educational purposes such as universities, institutions, and companies.
Al, B, C, Ce, Cr, Cu, Fe, Li, Mg, Mn, N, Nb, Nd, Ni, Si, Sn, Ti, V, Y, Zn, Zr

▲
Thermodynamic information for steel systems(Fe-Cr-Mo-Si-V-C) are provided as a free of charge to be used for educational purposes such as universities, institutions, and companies.
Cr < 30 | Mo < 10 | Si < 5 | V < 5 | C < 2
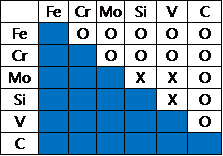
Thermodynamic information for steel systems(Fe-Cr-Mo-Si-V-C) are provided as a free of charge to be used for educational purposes such as universities, institutions, and companies.
Cr < 30 | Mo < 10 | Si < 5 | V < 5 | C < 2

C-Cr-Fe, C-Cr-V, C-Fe-Si, C-Fe-V, C-Fe-Mo, Cr-Fe-V
Some examples of what data can obtain by Calphad User-defined menu.
This example is useful when you want to know the state of each phase by temperature according to the amount of one element in a situation where different amounts of alloying elements are determined.
We will obtain the phase information by temperature according to the amount of Cr when 8wt.% Ni.
Select Fe, Cr, Ni
Select Steel database
Select All phase
Setting initial parameters
Major Element: Fe
Cr = 0.5, Ni = 0.08
Temp. = 1200 K
Mol/Weight: Weight
Select axis
For Axis 1, Type is fraction, Min = 0, Max = 0.9, increment = 0.01, Element = Cr
For Axis 2, Type is Temp., Min = 600, Max = 2000, increment = 10
Put Job name and click Start Job button
Select Steel database
Select All phase
Setting initial parameters
Major Element: Fe
Cr = 0.5, Ni = 0.08
Temp. = 1200 K
Mol/Weight: Weight
Select axis
For Axis 1, Type is fraction, Min = 0, Max = 0.9, increment = 0.01, Element = Cr
For Axis 2, Type is Temp., Min = 600, Max = 2000, increment = 10
Put Job name and click Start Job button
The result is:

This example is want to find out the solubility line of VC carbide in austenite at the 10Mo-4.5Cr-0.1Si HS steel when the temperature is 1300 K.
Select Fe, Cr, Mo, Si, V, C
Select Steel database
Select FCC_A1 only
Setting initial parameters
Major Element: Fe
Cr = 0.045, Mo = 0.1, Si = 0.001, V = 0.009, C = 0.009
Temp. = 1300 K
Mol/Weight: Weight
Select axis
For Axis 1, Type is fraction, Min = 0, Max = 0.015, increment = 0.0005, Element = V
For Axis 2, Type is fraction, Min = 0, Max = 0.01, increment = 0.0005, Element = C
Put the Job name and click Start Job button.
Select Steel database
Select FCC_A1 only
Setting initial parameters
Major Element: Fe
Cr = 0.045, Mo = 0.1, Si = 0.001, V = 0.009, C = 0.009
Temp. = 1300 K
Mol/Weight: Weight
Select axis
For Axis 1, Type is fraction, Min = 0, Max = 0.015, increment = 0.0005, Element = V
For Axis 2, Type is fraction, Min = 0, Max = 0.01, increment = 0.0005, Element = C
Put the Job name and click Start Job button.
The result is:

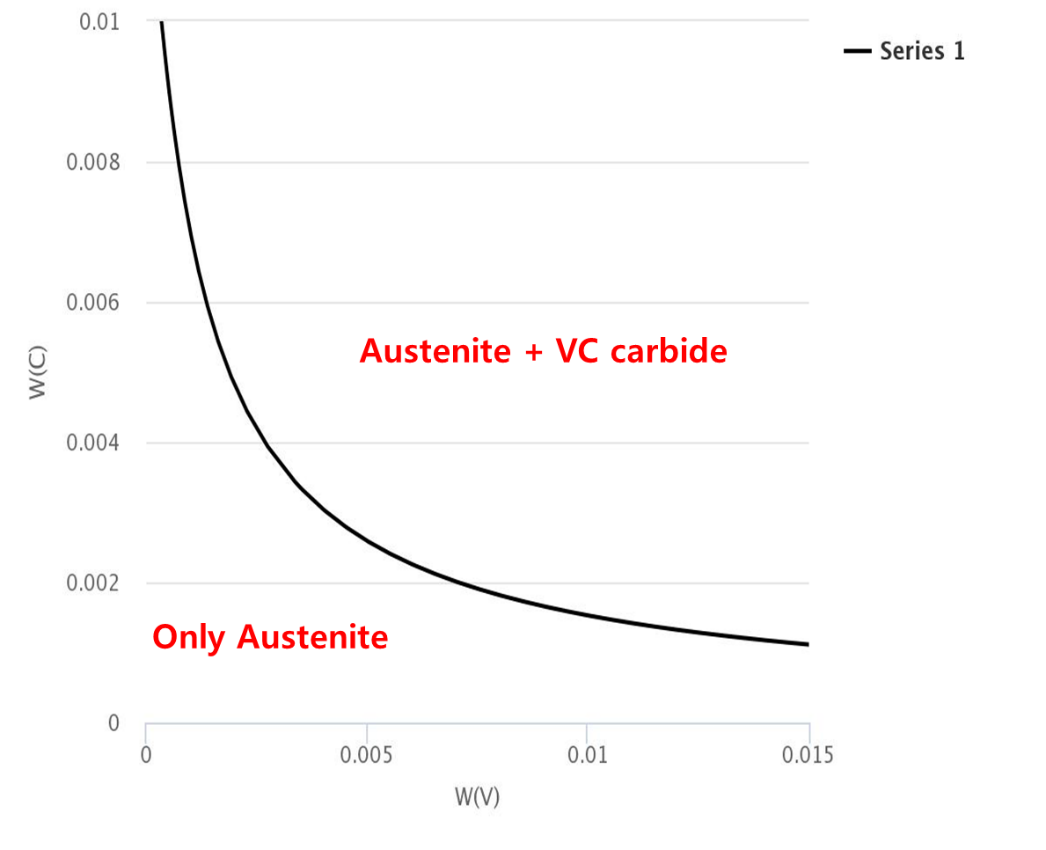
This result can analyze like the following figure by [List-equilibrium] tab.

We can check the crystallization amout of VC carbide exists in the Austenite according to how much the amount of V and C at the final.
| Type | Description | Unit |
|---|---|---|
| GM | Total gibbs energy for the system | J/mol |
| HM | Total enthalpy for the system | J/mol |
| SM | Total entropy for the system | J/mol·K |
| G | Total gibbs energy for the system | J |
| H | Total enthalpy for the system | J |
| S | Total entropy for the system | J/K |
| CP | Heat capacity for the system | J/K |
| GM(*) | The gibbs energy of individual phases | J/mol |
| HM(*) | The enthalpy of individual phases | J/mol |
| SM(*) | The entropy of individual phases | J/mol |
| G(*) | The gibbs energy of individual phases | J |
| H(*) | The enthalpy of individual phases | J |
| S(*) | The entropy of individual phases | J |
| AC(*) | The activity of individual elements | |
| Function | Custom mode |
This mode is used when you want to know the change of energy as the function of a specific phase or element.
- GM(FCC)
- GM(FCC)-GM(BCC)
- HM(FCC)-HM(BCC)
- SM(FCC)-SM(HCP)
- GM(FCC)-HM(FCC)
- GM(FCC)-HM(BCC)
- AC(Ni)
In this way, functions can also be computed each other.